Central Nervous System
Particle therapy, especially proton therapy, has been routinely performed for more than 30 years for various selective brain tumors as well as tumors of the skull base.
Particle therapy, i.e. proton and carbon ion radiotherapy, is primarily based on the better physical dose distribution, i.e. the radiation dose can be centered on the tumor, with a very fast decrease of the radiation dose towards the normal brain. Multiple comparisons with photon therapy have shown that the exposure of the normal brain to a low or moderate radiation dose can be significantly reduced with the use of protons and/or carbon ions. Even a relatively low radiation exposure of, however, larger parts of the normal brain can lead to long-term limitations of quality of life and neurocognitive functions. This is of course pronounced in childhood with a still developing brain and neurocognitive functions, which is why proton therapy is generally recommended for brain tumors. However, radiotherapy of the brain can also lead to considerable limitations in adult age, not only to the possible loss of specific functions, but also to general limitations of neurocognitive and intellectual resilience in professional but also social everyday life. In contrast, a prospective study at MedAustron showed preservation of all neurocognitive abilities for the entire group up to two years in >120 adult patients.
The advantage of particle therapy is not only evident in tumors that are exposed to relatively high radiation doses, but also in tumors that require relatively moderate doses (e.g. 50-54 Gy), but the volume to be irradiated is very irregularly shaped, especially towards the base of the skull.
Due to the sparing of normal brain tissue, particle therapy can also be used for dose escalation, i.e. to apply higher radiation doses to the tumor itself, in those tumors where local tumor control and thus generally the chances of survival are to be improved.
All patients are asked to participate in the registry study. This allows MedAustron to collect the data of the patients also in the context of aftercare, in order to be able to prove the effectiveness of particle therapy in the individual case and then also to be able to analyze the results (of course anonymized) for the whole group. Participation or non-participation in the registry study does not affect treatment in any way.
The majority of pediatric and many adult CNS tumors requiring radiotherapy can be treated with particle therapy.
Proton therapy is part of the standard of care for the majority of pediatric brain tumors.
Increasingly, adult patients are also being assigned to particle therapy for specific indications.
Primary irradiations:
Low-grade gliomas (WHO I-III gliomas with evidence of an IDH1,2 mutation) but also other rare tumors within the CNS or in the direct vicinity such as pinaelis tumors, craniopharyngiomas, pituitary macroadenomas, ependymomas, schwannomas of the cranial nerves (e.g.: acoustic neuroma/vestibular schwannoma), meningiomas, hemangiopericytomas/solitary fibrous tumor, hemangioblastomas, and others.
According to the current state of science, high-grade gliomas (glioblastoma multiforme, grade IV, IDH neg. gliomas) benefit from particle therapy only in a few exceptions and highly individualized indications.
Re-irradiation:
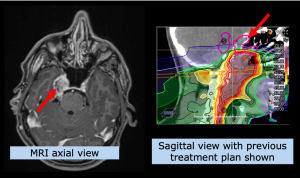
Despite initially successful irradiation with photons (but possibly also after particle therapy), a certain percentage of patients may experience a recurrence of the tumor in the previous irradiation area or in the immediate vicinity.
The therapeutic procedure is discussed on a multi-disciplinary basis, i.e. whether the recurrence should best be removed surgically, whether systemic therapy, such as chemotherapy or immunotherapy, should be used, or whether irradiation should be applied again. If another course of irradiation is considered, either as a sole therapy or in combination, the question arises to what extent another full course of irradiation can be given, due to the prior exposure of normal tissue. In other words, do the brain structures, such as normal brain parenchyma or even important nerves, allow another radiation exposure in order to be able to treat this tumor with a necessary radiation dose?
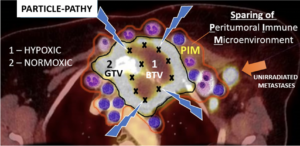
Here, particle therapy can play a decisive role in enabling re-irradiation, which cannot be offered by conventional radiotherapy, i.e. many radiation oncologists justifiably deny re-irradiation with photons. However, a therapeutic decision is highly individual. In order to be able to make such a decision, a detailed review is required, especially of the irradiation plan that has already been applied.
MedAustron has developed various concepts involving either proton or carbon ion radiotherapy or a combination. Whether re-irradiation with particle therapy can be performed, and if so, with which concept, is decided by the medical team at MedAustron on an individual basis and discussed with the patient.
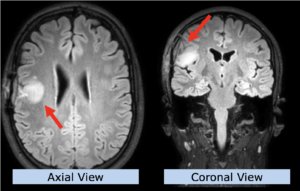
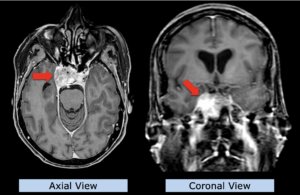
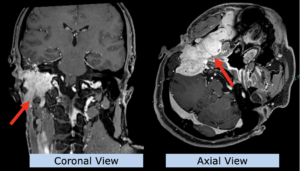
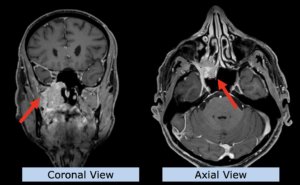
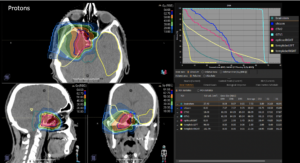
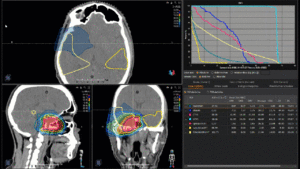
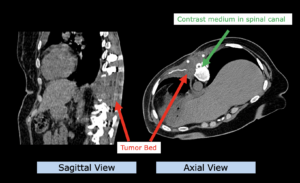
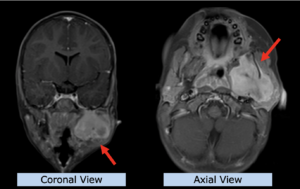
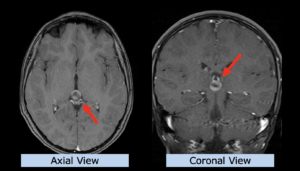
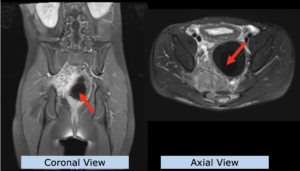
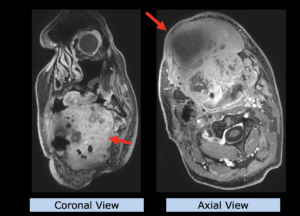
22-year-old female patient with a low grade glioma (WHO II, IDH mut) in the left opercular region of the left hemisphere.
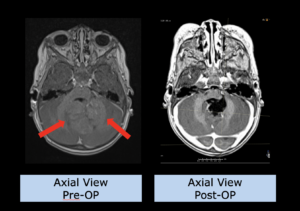
Underwent biopsy and partial resection. Complete surgery not feasible. Sent for Proton Therapy.
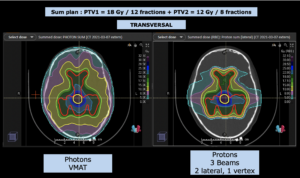
Treated according to institutional protocol protocol to total dose of 54 Gy (RBE).
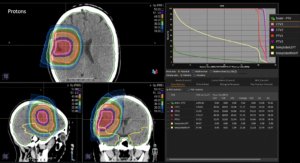
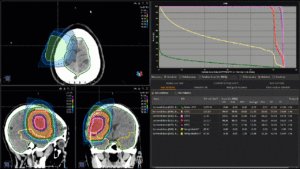
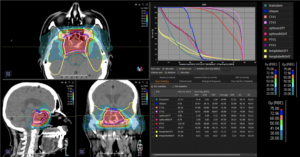
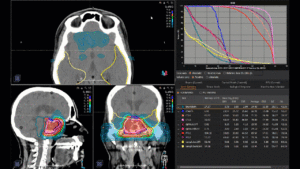
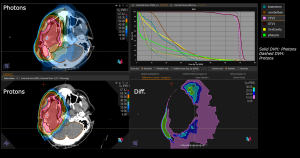
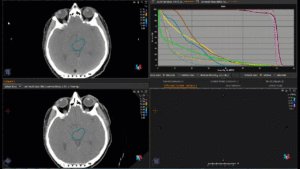
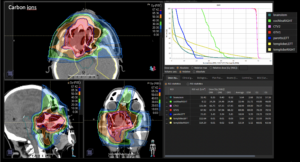
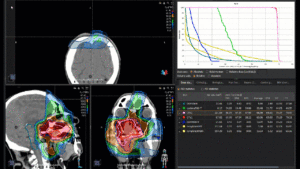
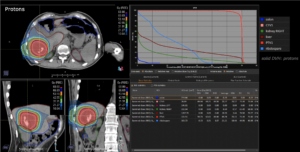
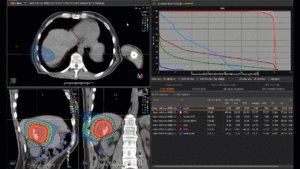
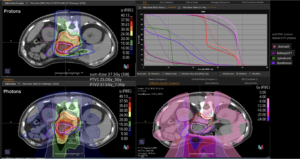
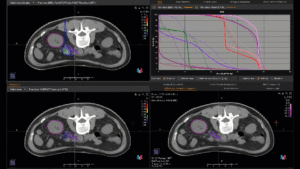
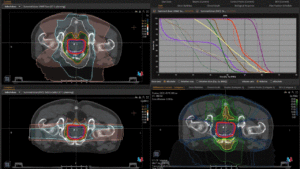
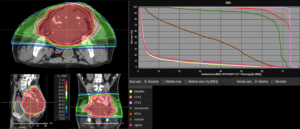
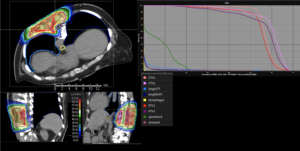
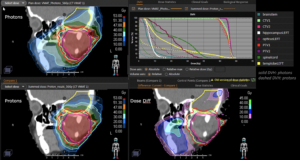
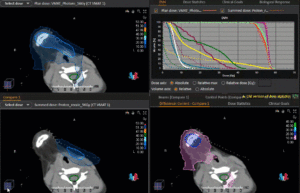
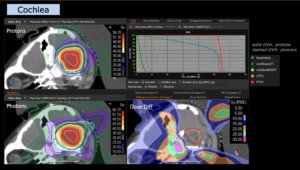
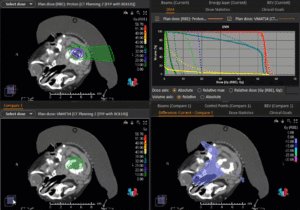
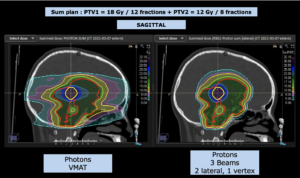
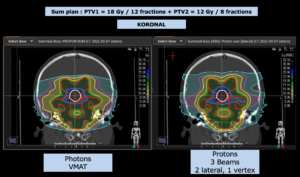
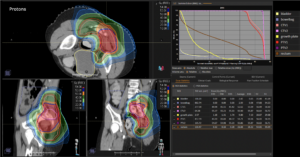
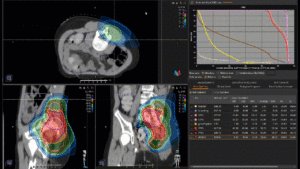
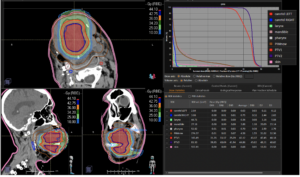
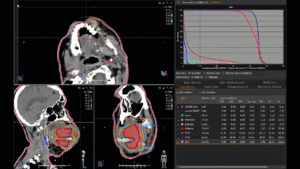
Graph and video demonstrating the full irradiation plan using proton therapy:
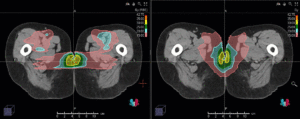
Left side: Proton plan in 3 views: axial, sagittal (side) and coronal (frontal view).
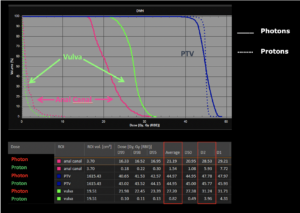
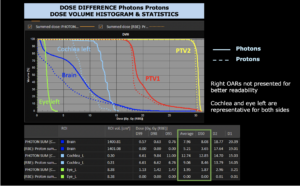
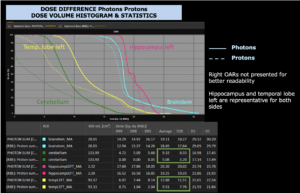
Right side: dose/volume histograms (DVH‘s), meaning the graphic distribution of dose delivered to the volume of specific organs – in this case brain and temporal lobes. In addition numerical display of various dose levels.
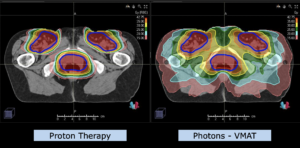
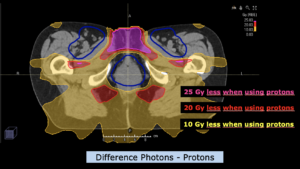
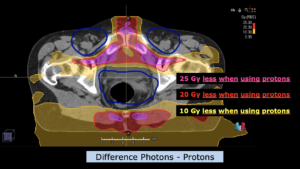
Note: Of clinical importance was achieving an optimal tumor coverage, despite not exceeding doses to the critical organs located in direct proximity: the non-involved healthy CNS parenchyma especially the temporal lobe on both sides. Note the complete absence of dose to the contralateral brain.
The excellent dose distribution is expected to result in a low risk of brain toxicity and low risk of decline of cognitive function.
In general, there are no side effects specific to particle therapy, but side effects may occur, as with conventional photon therapy, too. Since less dose reaches the surrounding normal tissue due to the physical properties described, these side effects can be significantly less pronounced.
Because CNS tissue can be very sensitive to ionizing radiation (radiotherapy) and thus be damaged in the long term. One of the main causes is damage to the smallest blood vessels/capillaries, which in turn leads in the long term to a reduction in blood circulation and corresponding damage to the tissue. These effects can also be observed in areas that received only a low dose.
Clinically, this can lead to neurocognitive changes and mental slowing. Further side effects of radiotherapy, depending on the localization, can be neurological changes up to deficits (hearing, visual acuity, sense of taste/smell,…) as well as hormonal changes / deficits if the pituitary gland is involved. All of these effects can occur years after radiotherapy and affect quality of life. The risk of these late effects can be significantly reduced with proton therapy.
a. Can particle therapy be combined with systemic therapy?
i. Depending on the tumor, particle therapy can be combined with systemic therapy, as it is with conventional radiotherapy as well. In such cases, the indication is made on an interdisciplinary basis (medical oncologist and radiation oncologist).
b. Is particle therapy possible after surgery as well?
i. Particle therapy can also be performed after surgery, especially if the surgeon could not remove everything, it is also indicated.
c. Is there a limitation on the tumor size in particle therapy?
i. In principle, tumors of any size can be treated with particle therapy.
d. Can particle therapy replace surgery?
i. If a tumor is accessible to surgery, this is the treatment of choice in the majority of cases. In some situations, surgery is associated with high risk, so that irradiation can be performed as an option with curative intent.
e. Can particle therapy cause side effects?
i. As with conventional irradiation, local reactions can also occur in the surrounding normal tissue – but as a rule these are much less pronounced, since due to the physical properties this tissue is exposed to a lower dose.
f. Are the chances of cure higher after particle therapy than after conventional irradiation?
i. The biological effect of proton therapy is very similar to that of photon therapy; thus, the chances of cure at the same dose are identical after proton therapy as after conventional irradiation. Due to the lower normal tissue exposure, in special situations the total dose can be increased during proton therapy and consequently an improvement in local control can be achieved.
g. Are there age limits for particle therapy?
i. In principle, patients of any age who are indicated for radiotherapy can be treated with particle therapy.
1. Superior Intellectual Outcomes After Proton Radiotherapy Compared With Photon Radiotherapy for Pediatric Medulloblastoma. Kahalley LS, Peterson R, Ris MD, et al. J Clin Oncol. 2020 Feb 10;38(5):454-461.
2. Proton therapy for selected low grade glioma patients in the Netherlands. van der Weide HL, Kramer MCA, Scandurra D, et al. Radiother Oncol. 2021 Jan;154:283-290.
3. Proton therapy for treatment of intracranial benign tumors in adults: A systematic review. Lesueur P, Calugaru V, Nauraye C, et al. Cancer Treat Rev. 2019 Jan;72:56-64.
4. Proton Therapy for Intracranial Meningioma for the Treatment of Primary/Recurrent Disease Including Re-Irradiation. Weber DC, Bizzocchi N, Bolsi A, et al. Front Oncol. 2020 Dec 14;10:558845.
5. A Multi-institutional Comparative Analysis of Proton and Photon Therapy-Induced Hematologic Toxicity in Patients With Medulloblastoma. Liu KX, Ioakeim-Ioannidou M, Susko MS, et al. Int J Radiat Oncol Biol Phys. 2021 Mar 1;109(3):726-735.
6. A Systematic Review on Re-irradiation with Charged Particle Beam Therapy in the Management of Locally Recurrent Skull Base and Head and Neck Tumors. Gamez ME, Patel SH, McGee LA, et al. Int J Part Ther. 2021 Jun 25;8(1):131-154.
7. Re-irradiation with protons or heavy ions with focus on head and neck, skull base and brain malignancies. Seidensaal K, Harrabi SB, Uhl M, et al. Br J Radiol. 2020 Mar;93(1107):20190516